| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
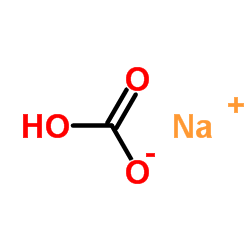 |
SodiuM bicarbonate
CAS:144-55-8 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
Phenol
CAS:108-95-2 |
|
 |
oleic acid
CAS:112-80-1 |
|
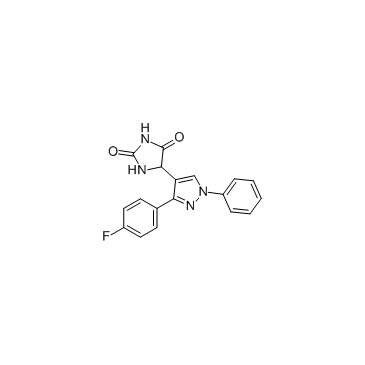 |
DPH
CAS:484049-04-9 |
|
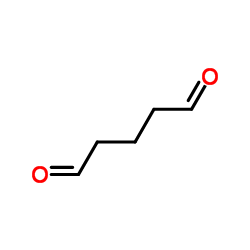 |
glutaraldehyde
CAS:111-30-8 |
|
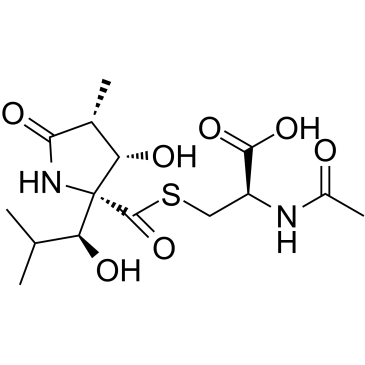 |
Lactacystin
CAS:133343-34-7 |
|
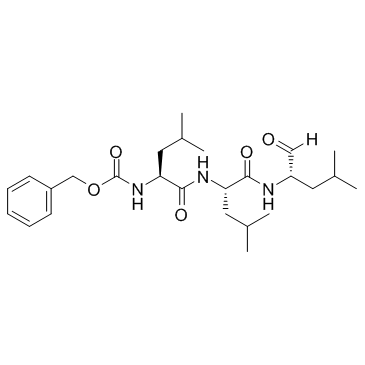 |
MG-132
CAS:133407-82-6 |
|
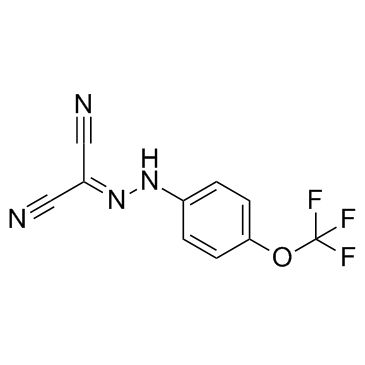 |
FCCP
CAS:370-86-5 |