| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
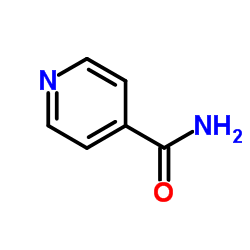 |
Isonicotinamide
CAS:1453-82-3 |
|
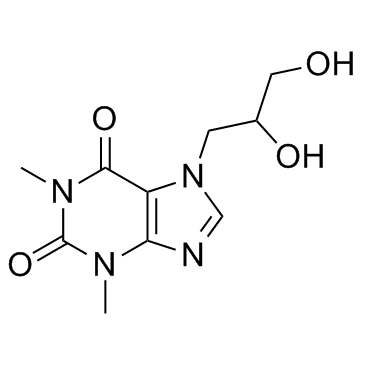 |
Diprophylline
CAS:479-18-5 |