| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
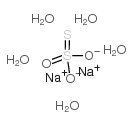 |
sodium thiosulfate pentahydrate
CAS:10102-17-7 |
|
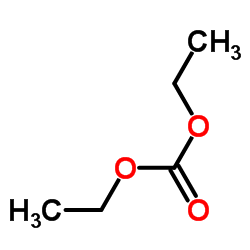 |
Ethyl carbonate
CAS:105-58-8 |
|
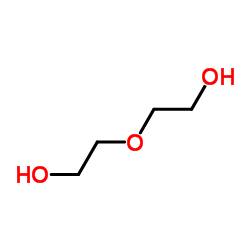 |
Diethylene glycol
CAS:111-46-6 |
|
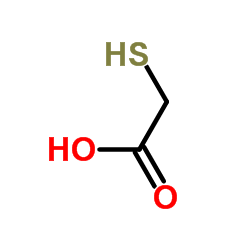 |
Mercaptoacetic acid
CAS:68-11-1 |