| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
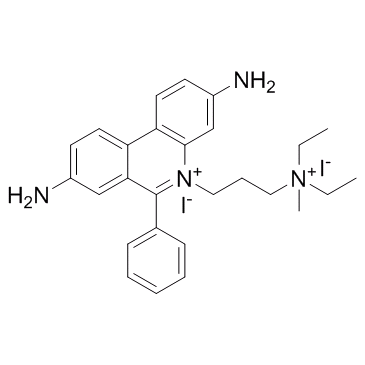 |
Propidium Iodide
CAS:25535-16-4 |
|
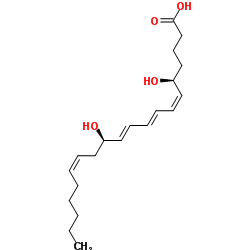 |
Leukotriene B4
CAS:71160-24-2 |