| Structure | Name/CAS No. | Articles |
|---|---|---|
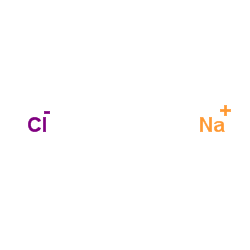 |
sodium chloride
CAS:7647-14-5 |
|
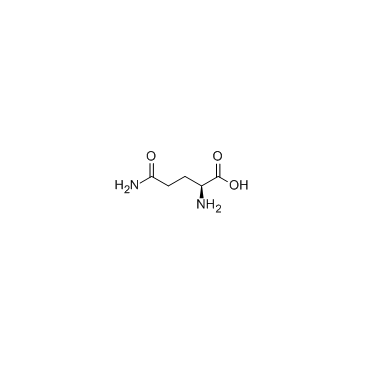 |
L-Glutamine
CAS:56-85-9 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
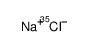 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
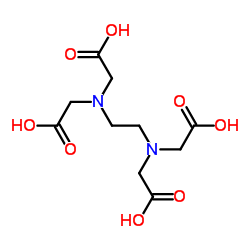 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
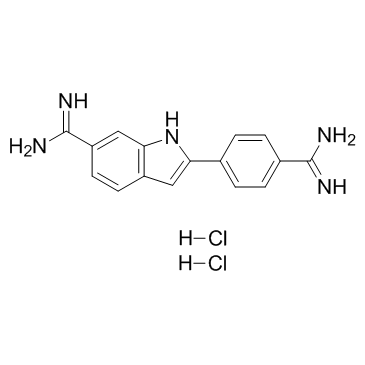 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
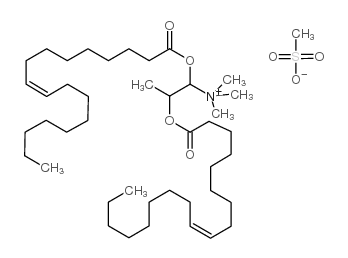 |
DOTAP Transfection Reagent
CAS:144189-73-1 |
|
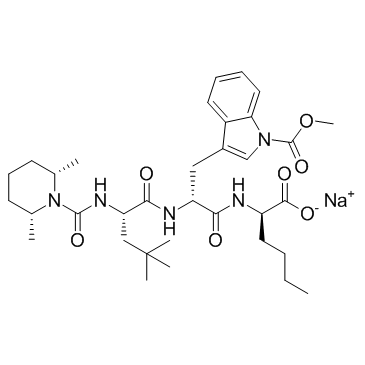 |
BQ-788 (sodium salt)
CAS:156161-89-6 |
|
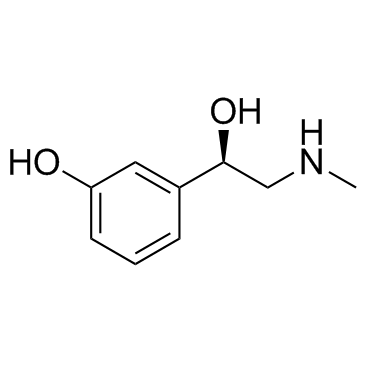 |
(R)-(-)-Phenylephrine
CAS:59-42-7 |