| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
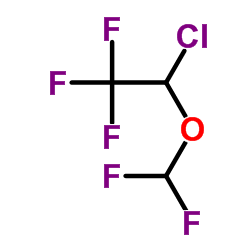 |
Isoflurane
CAS:26675-46-7 |
|
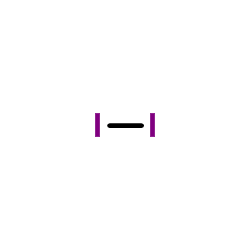 |
molecular iodine
CAS:7553-56-2 |
|
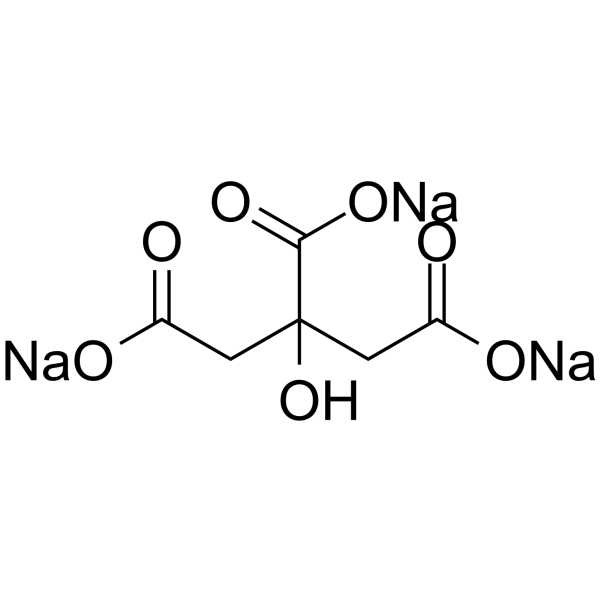 |
Sodium citrate
CAS:68-04-2 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
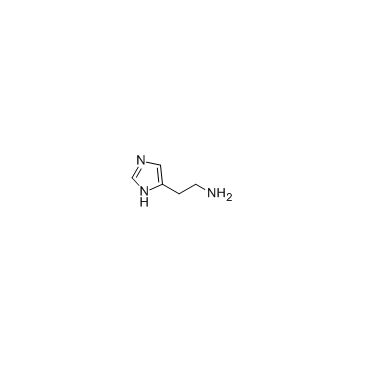 |
Histamine
CAS:51-45-6 |
|
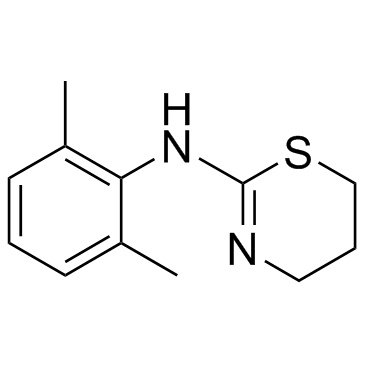 |
Xylazine
CAS:7361-61-7 |
|
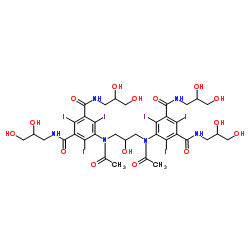 |
Iodixanol
CAS:92339-11-2 |
|
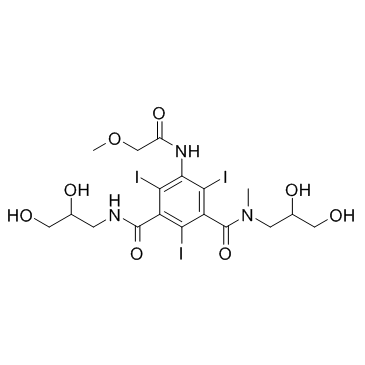 |
iopromide
CAS:73334-07-3 |