| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
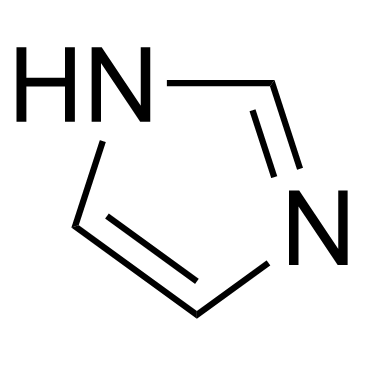 |
Imidazole
CAS:288-32-4 |
|
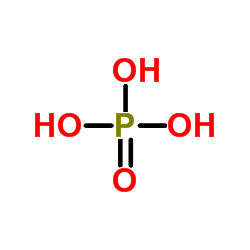 |
Phosphoric acid
CAS:7664-38-2 |
|
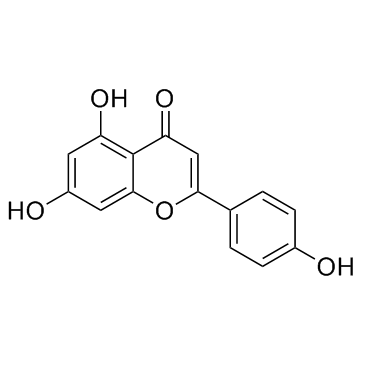 |
Apigenin
CAS:520-36-5 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
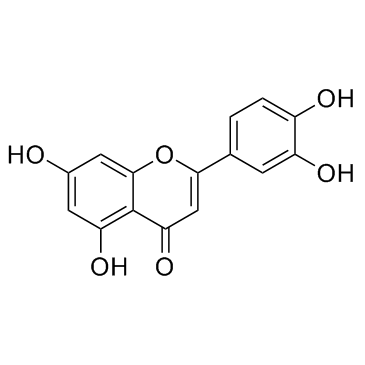 |
Luteolin
CAS:491-70-3 |
|
 |
udp-alpha-d-galactose disodium salt
CAS:137868-52-1 |
|
 |
(±)-Naringenin
CAS:67604-48-2 |
|
 |
trisodium phosphate
CAS:7601-54-9 |
|
 |
Quercetin
CAS:117-39-5 |