| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
Formaldehyde
CAS:50-00-0 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
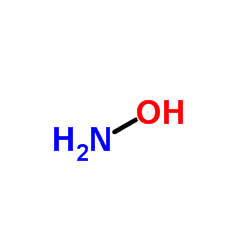 |
Hydroxylamine
CAS:7803-49-8 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
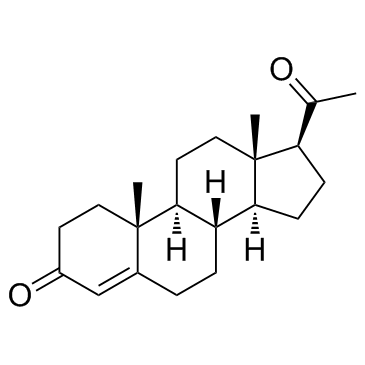 |
Progesterone
CAS:57-83-0 |
|
 |
Hydrocortisone
CAS:50-23-7 |
|
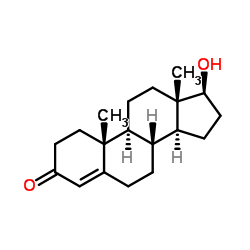 |
Testosterone
CAS:58-22-0 |
|
 |
Pregnenolone
CAS:145-13-1 |