| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Formic Acid
CAS:64-18-6 |
|
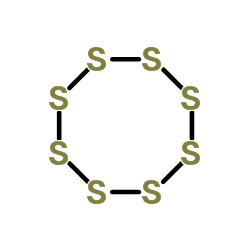 |
Sulfur
CAS:7704-34-9 |
|
 |
2-Chloroethyl ethyl sulfide
CAS:693-07-2 |
|
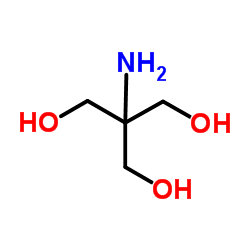 |
Trometamol
CAS:77-86-1 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
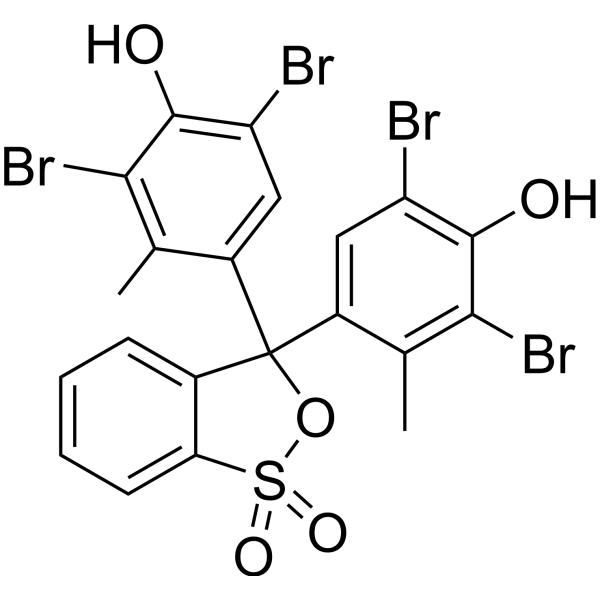 |
Bromocresol Green
CAS:76-60-8 |
|
 |
trisodium phosphate
CAS:7601-54-9 |