| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sucrose
CAS:57-50-1 |
|
 |
Calcium chloride
CAS:10043-52-4 |
|
 |
Osmium tetroxide
CAS:20816-12-0 |
|
 |
EGTA
CAS:67-42-5 |
|
 |
Thapsigargin
CAS:67526-95-8 |
|
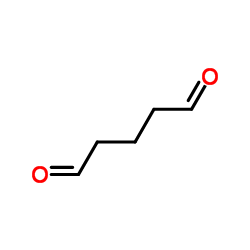 |
glutaraldehyde
CAS:111-30-8 |
|
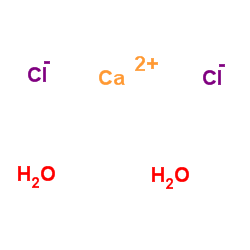 |
calcium chloride dihydrate
CAS:10035-04-8 |
|
 |
Insulin(human)
CAS:11061-68-0 |