| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
N-hexane
CAS:110-54-3 |
|
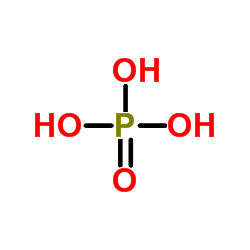 |
Phosphoric acid
CAS:7664-38-2 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
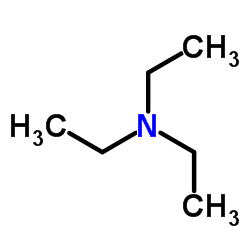 |
Triethylamine
CAS:121-44-8 |
|
 |
Ammonia
CAS:7664-41-7 |
|
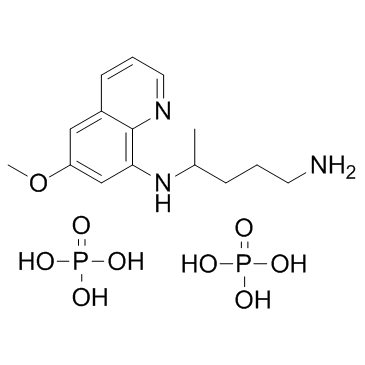 |
primaquine phosphate
CAS:63-45-6 |
|
 |
AMMONIA (14N)
CAS:1026405-88-8 |