| Structure | Name/CAS No. | Articles |
|---|---|---|
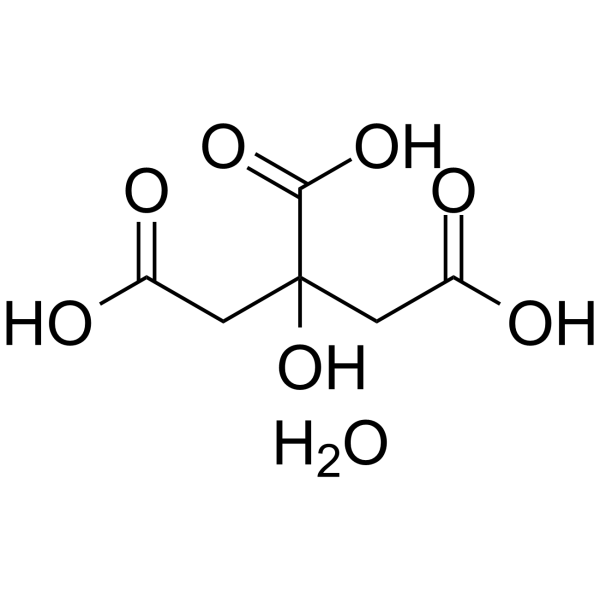 |
Citric acid monohydrate
CAS:5949-29-1 |
|
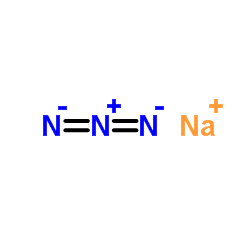 |
Sodium azide
CAS:26628-22-8 |
|
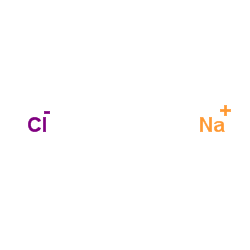 |
sodium chloride
CAS:7647-14-5 |
|
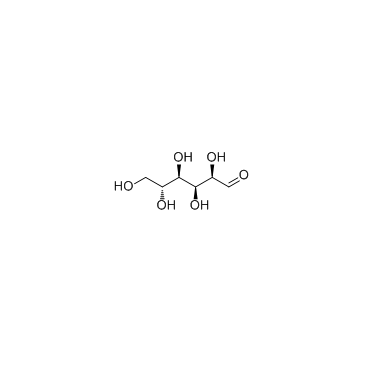 |
D-(+)-Glucose
CAS:50-99-7 |
|
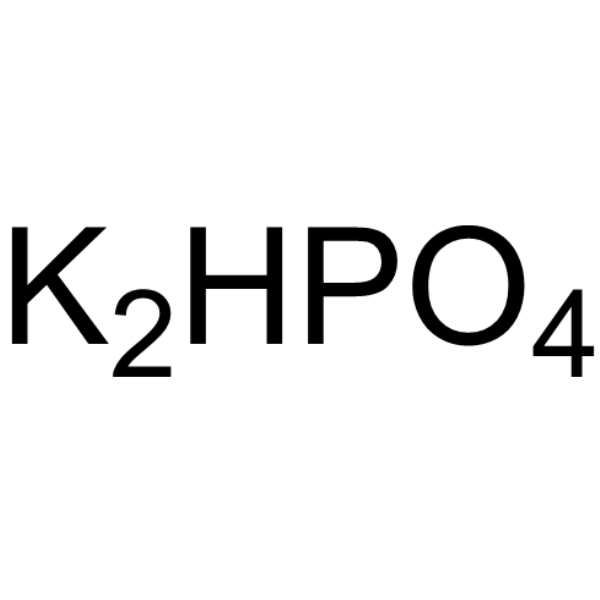 |
Di-potassium monohydrogen phosphate
CAS:7758-11-4 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
 |
sodium dihydrogenphosphate
CAS:7558-80-7 |
|
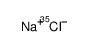 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
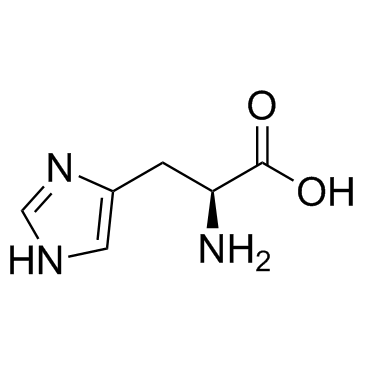 |
L-Histidine
CAS:71-00-1 |
|
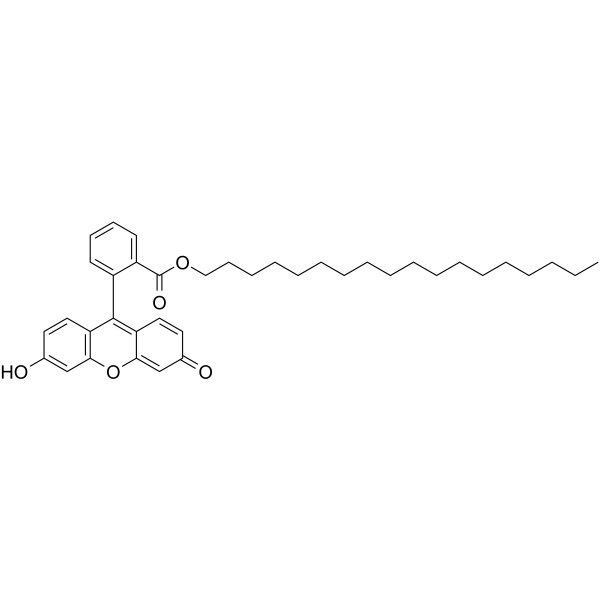 |
chromoionophore ii
CAS:138833-46-2 |