| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
bisphenol A
CAS:80-05-7 |
|
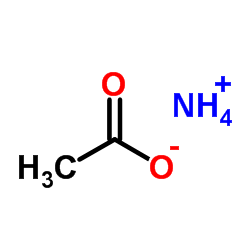 |
Ammonium acetate
CAS:631-61-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Formaldehyde
CAS:50-00-0 |
|
 |
Hexanoic acid,2-hydroxy
CAS:6064-63-7 |
|
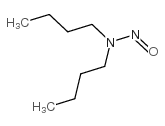 |
N-Nitrosodibutylamine
CAS:924-16-3 |
|
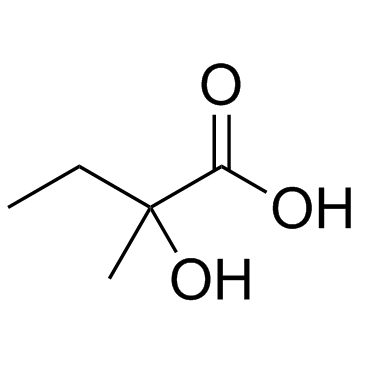 |
2-Hydroxy-2-methylbutyric acid
CAS:3739-30-8 |
|
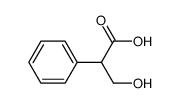 |
Tropic acid
CAS:552-63-6 |
|
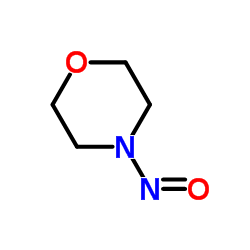 |
4-Nitrosomorpholine
CAS:59-89-2 |
|
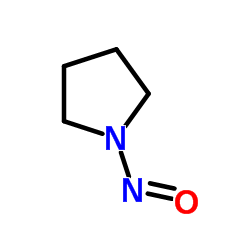 |
NPYR
CAS:930-55-2 |