| Structure | Name/CAS No. | Articles |
|---|---|---|
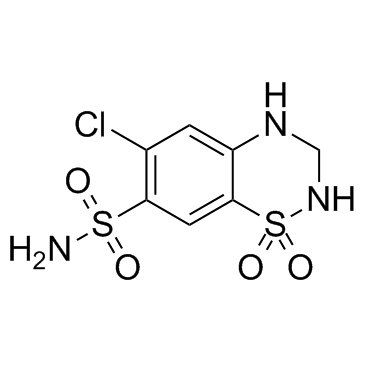 |
Hydrochlorothiazide
CAS:58-93-5 |
|
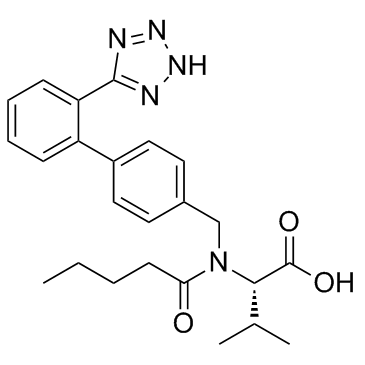 |
Valsartan
CAS:137862-53-4 |
|
 |
Amlodipine besylate
CAS:111470-99-6 |