| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
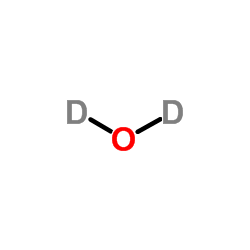 |
Heavy water
CAS:7789-20-0 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Acetone
CAS:67-64-1 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
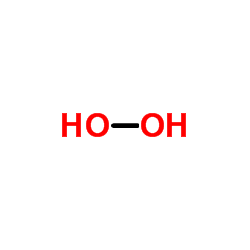 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
methylamine
CAS:74-89-5 |