| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Isopropanol
CAS:67-63-0 |
|
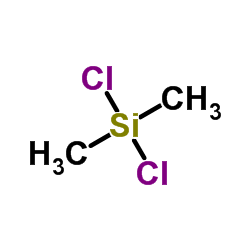 |
Dichlorodimethylsilane
CAS:75-78-5 |
|
 |
cyclohexane
CAS:110-82-7 |