| Structure | Name/CAS No. | Articles |
|---|---|---|
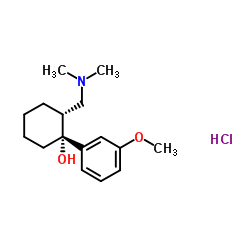 |
Tramadol hydrochloride
CAS:36282-47-0 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
ethyl acetate
CAS:141-78-6 |