| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Cyclosporin A
CAS:59865-13-3 |
|
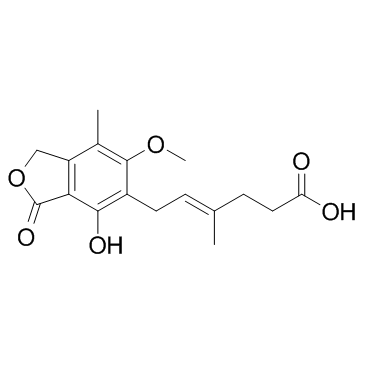 |
Mycophenolic acid
CAS:24280-93-1 |
|
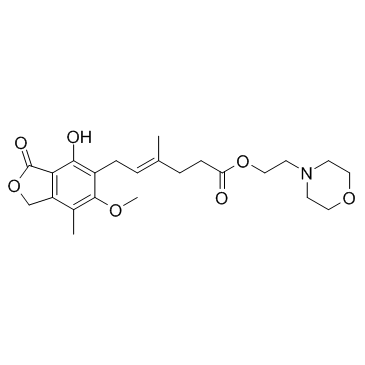 |
Mycophenolate mofetil
CAS:128794-94-5 |