| Structure | Name/CAS No. | Articles |
|---|---|---|
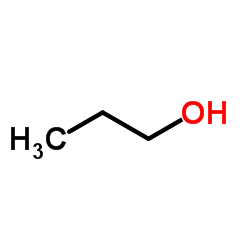 |
1-Propanol
CAS:71-23-8 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
N-hexane
CAS:110-54-3 |
|
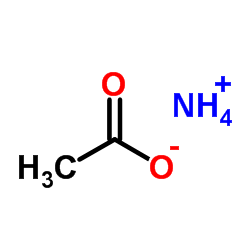 |
Ammonium acetate
CAS:631-61-8 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
acetic acid
CAS:64-19-7 |
|
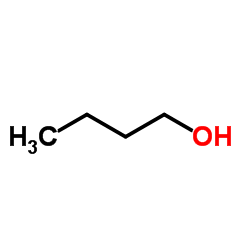 |
Butanol
CAS:71-36-3 |