| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Chloroform
CAS:67-66-3 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Water
CAS:7732-18-5 |
|
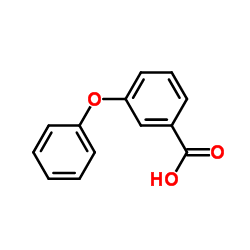 |
3-Phenoxybenzoic acid
CAS:3739-38-6 |
|
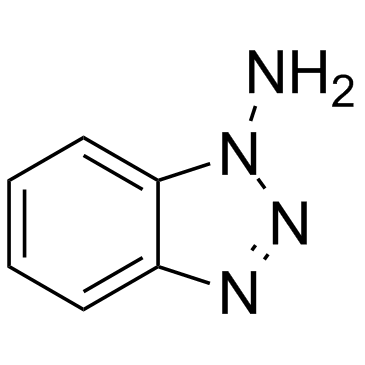 |
1-Aminobenzotriazole
CAS:1614-12-6 |