| Structure | Name/CAS No. | Articles |
|---|---|---|
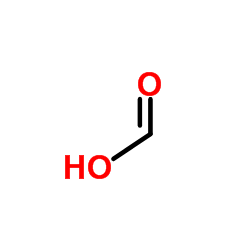 |
Formic Acid
CAS:64-18-6 |
|
 |
sucrose
CAS:57-50-1 |
|
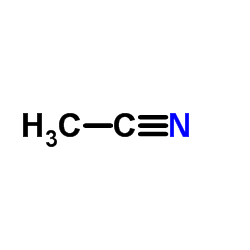 |
Acetonitrile
CAS:75-05-8 |
|
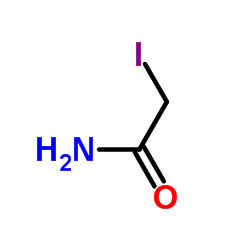 |
Iodoacetamide
CAS:144-48-9 |
|
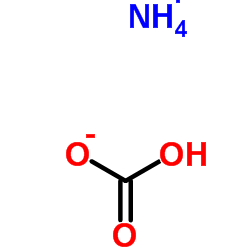 |
Ammonium Bicarbonate
CAS:1066-33-7 |
|
 |
potassium chloride
CAS:7447-40-7 |
|
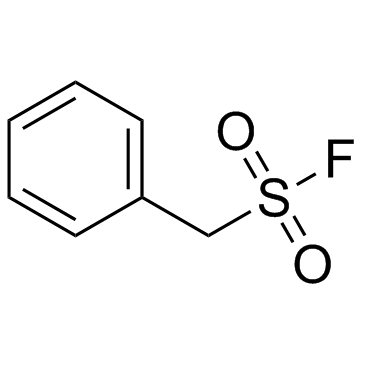 |
PMSF
CAS:329-98-6 |
|
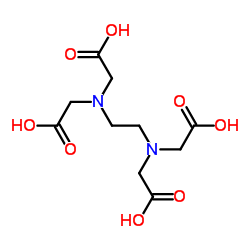 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
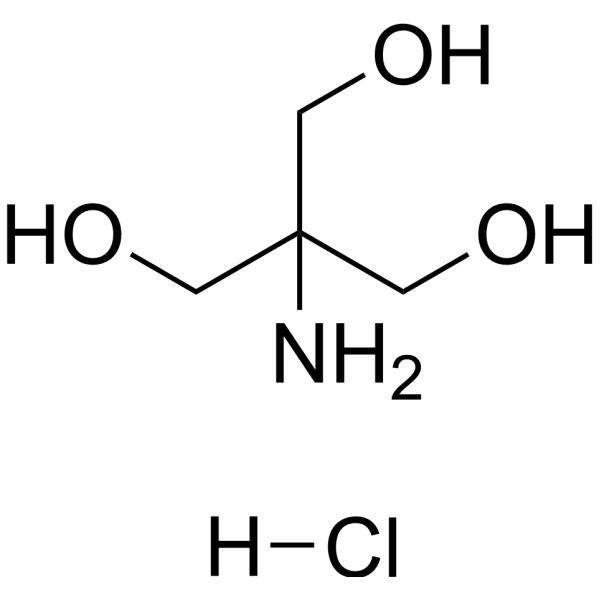 |
Trometamol hydrochloride
CAS:1185-53-1 |
|
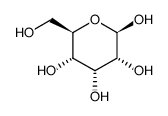 |
Beta-D-allose
CAS:7283-09-2 |