| Structure | Name/CAS No. | Articles |
|---|---|---|
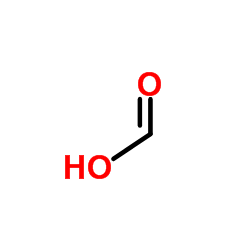 |
Formic Acid
CAS:64-18-6 |
|
 |
Lithium acetate hydrate (1:1:2)
CAS:6108-17-4 |
|
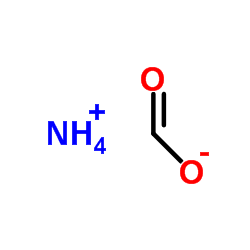 |
Formic acid ammonium salt
CAS:540-69-2 |
|
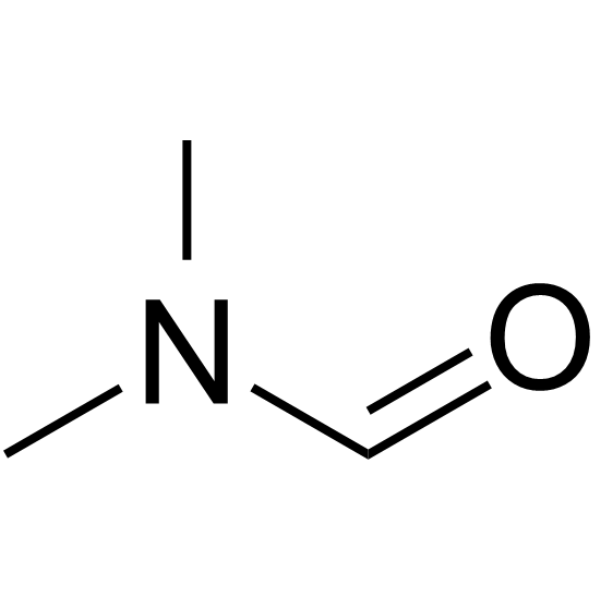 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
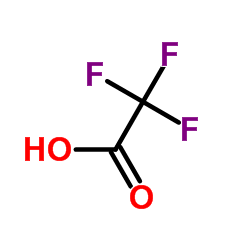 |
trifluoroacetic acid
CAS:76-05-1 |
|
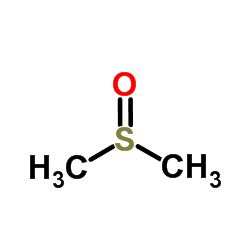 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
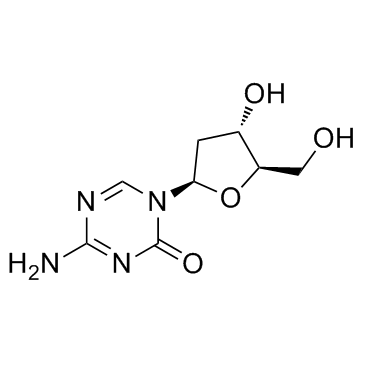 |
Decitabine
CAS:2353-33-5 |
|
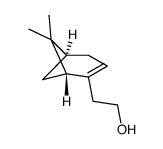 |
(-)-NOPOL
CAS:35836-73-8 |
|
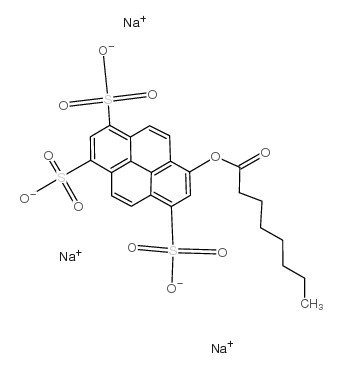 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |