| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
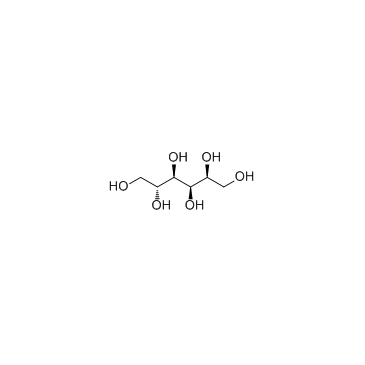 |
Sorbitol
CAS:50-70-4 |
|
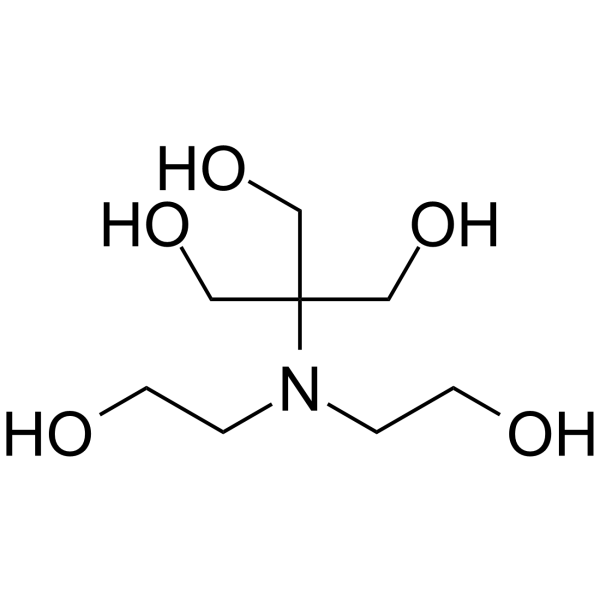 |
Bis-tris methane
CAS:6976-37-0 |
|
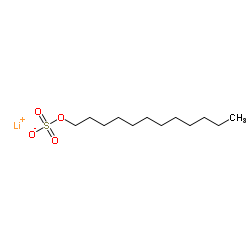 |
Lithium dodecyl sulfate
CAS:2044-56-6 |
|
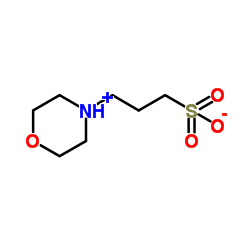 |
MOPS
CAS:1132-61-2 |
|
 |
trisodium phosphate
CAS:7601-54-9 |