| Structure | Name/CAS No. | Articles |
|---|---|---|
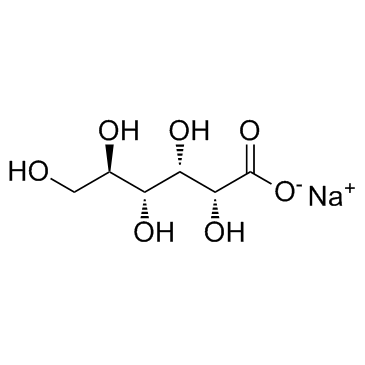 |
Sodium Gluconate
CAS:527-07-1 |
|
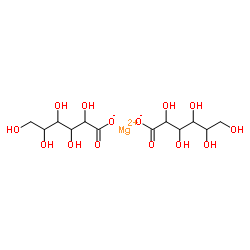 |
Magnesium dihexonate
CAS:3632-91-5 |
|
 |
L-cysteine
CAS:52-90-4 |
|
 |
4-Aminobutanoic acid
CAS:56-12-2 |
|
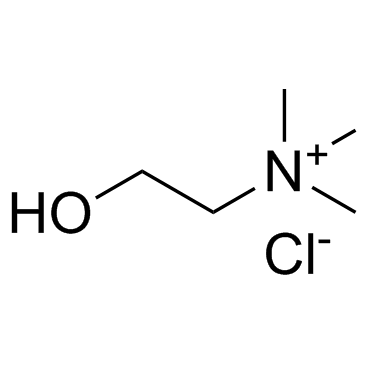 |
Choline chloride
CAS:67-48-1 |
|
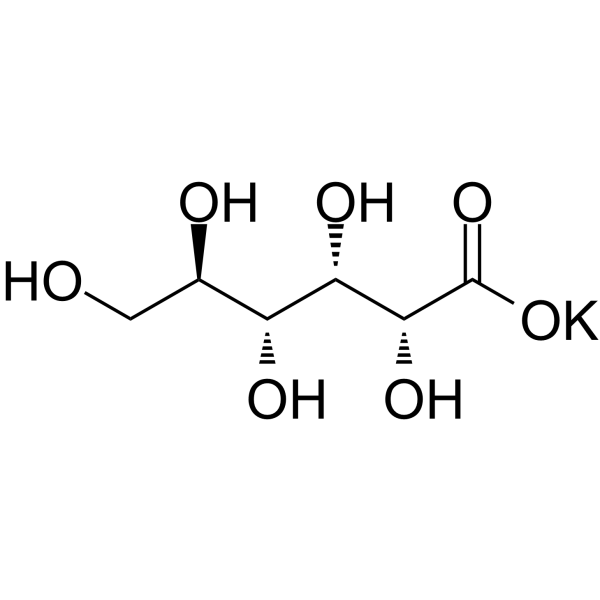 |
Potassium Gluconate
CAS:299-27-4 |