| Structure | Name/CAS No. | Articles |
|---|---|---|
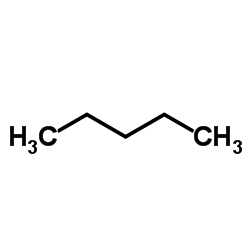 |
Pentane
CAS:109-66-0 |
|
 |
Ethanol
CAS:64-17-5 |
|
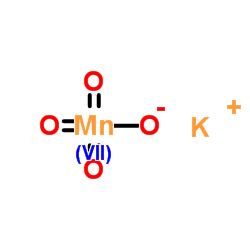 |
Potassium permanganate
CAS:7722-64-7 |
|
 |
Methanol
CAS:67-56-1 |
|
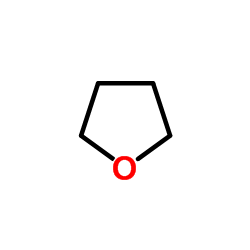 |
thf
CAS:109-99-9 |
|
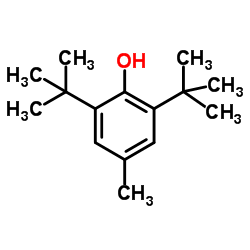 |
Butylated hydroxytoluene
CAS:128-37-0 |
|
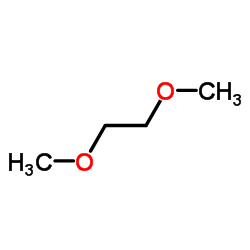 |
1,2-dimethoxyethane
CAS:110-71-4 |