| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium carbonate
CAS:497-19-8 |
|
 |
Acetone
CAS:67-64-1 |
|
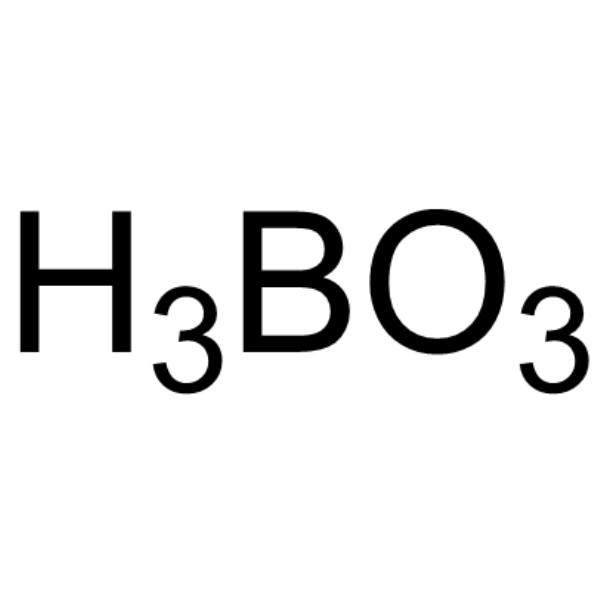 |
Orthoboric acid
CAS:10043-35-3 |
|
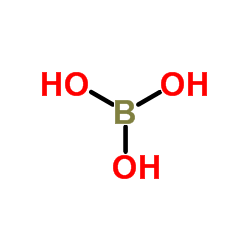 |
Boric acid-11B
CAS:13813-78-0 |
|
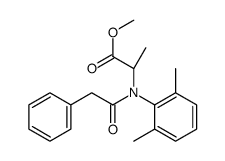 |
Benalaxyl-M
CAS:98243-83-5 |
|
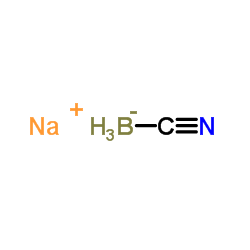 |
Sodium cyanoborohydride
CAS:25895-60-7 |
|
 |
N-(3-Aminopropyl)methacrylamide hydrochloride
CAS:72607-53-5 |