| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
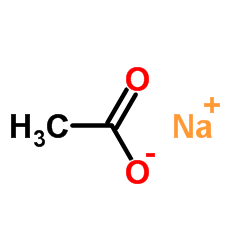 |
Sodium acetate
CAS:127-09-3 |
|
 |
Cyclosporin A
CAS:59865-13-3 |
|
 |
Formaldehyde
CAS:50-00-0 |
|
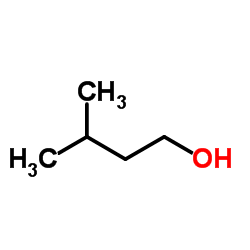 |
3-Methyl-1-butanol
CAS:123-51-3 |
|
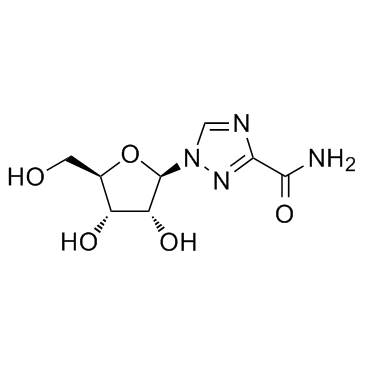 |
Ribavirin
CAS:36791-04-5 |
|
 |
(+/-)-2-HYDROXYTRIDECANOICACID
CAS:100828-16-8 |