| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
 |
sodiumborohydride
CAS:16940-66-2 |
|
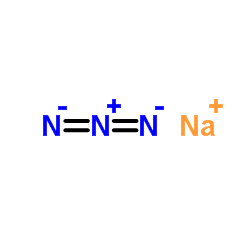 |
Sodium azide
CAS:26628-22-8 |
|
 |
Diethyl ether
CAS:60-29-7 |
|
 |
N-hexane
CAS:110-54-3 |
|
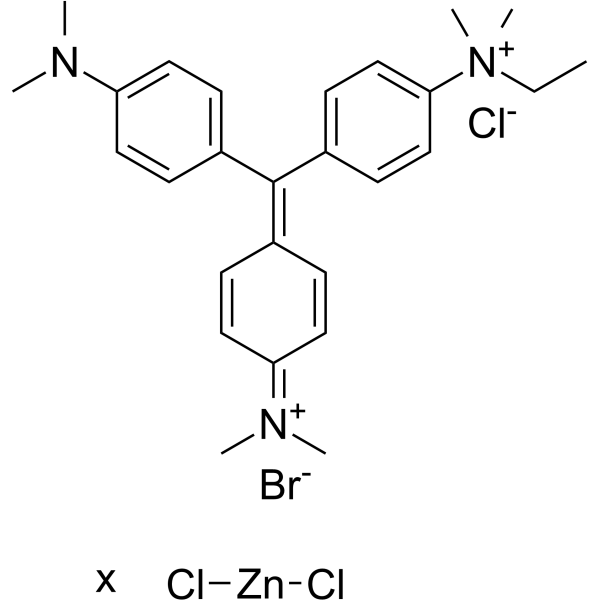 |
Methyl green
CAS:7114-03-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
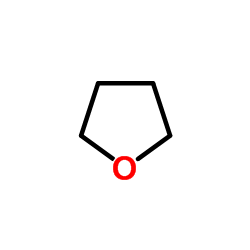 |
thf
CAS:109-99-9 |
|
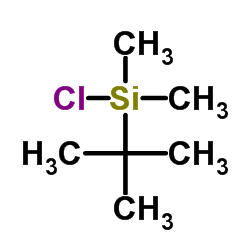 |
tert-Butyldimethylsilyl chloride
CAS:18162-48-6 |