| Structure | Name/CAS No. | Articles |
|---|---|---|
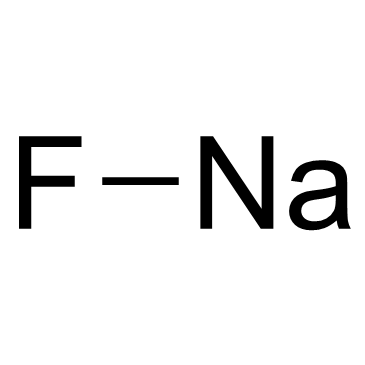 |
Sodium Fluoride
CAS:7681-49-4 |
|
 |
sucrose
CAS:57-50-1 |
|
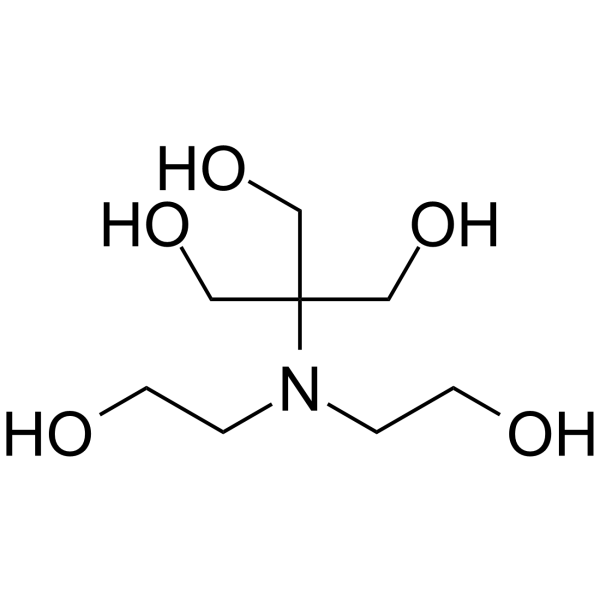 |
Bis-tris methane
CAS:6976-37-0 |
|
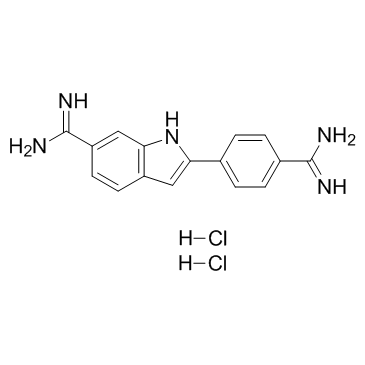 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
 |
3-Methyladenine
CAS:5142-23-4 |
|
 |
N,N′-diisopropylcarbodiimide
CAS:693-13-0 |
|
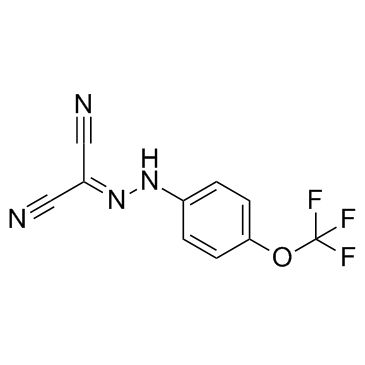 |
FCCP
CAS:370-86-5 |
|
 |
DL-Dithiothreitol
CAS:3483-12-3 |