| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
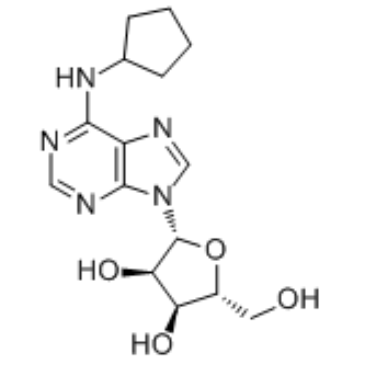 |
N6-Cyclopentyladenosine
CAS:41552-82-3 |
|
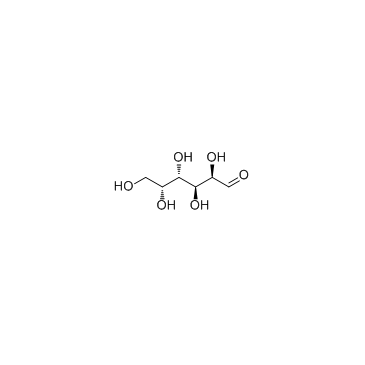 |
D-Galactose
CAS:59-23-4 |
|
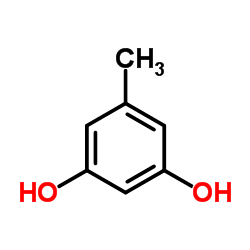 |
Orcinol
CAS:504-15-4 |