| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
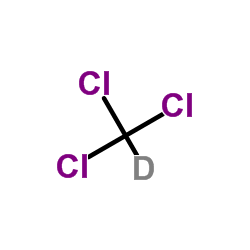 |
chloroform-d
CAS:865-49-6 |
|
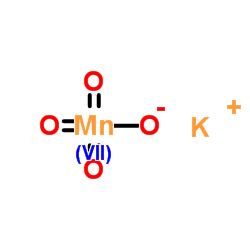 |
Potassium permanganate
CAS:7722-64-7 |
|
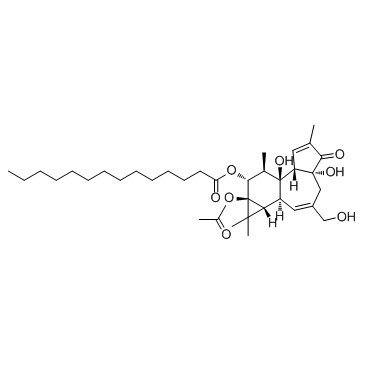 |
12-O-tetradecanoylphorbol-13-acetate
CAS:16561-29-8 |