| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
硫酸
CAS:7664-93-9 |
|
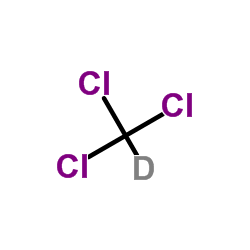 |
氘代氯仿-d
CAS:865-49-6 |
|
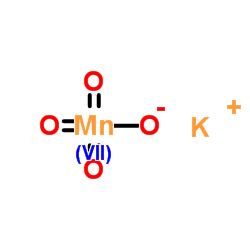 |
高锰酸钾
CAS:7722-64-7 |
|
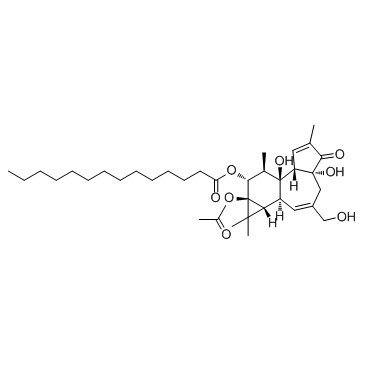 |
12-O-十四烷酰佛波醋酸酯-13
CAS:16561-29-8 |