| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
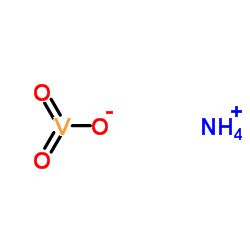 |
Ammonium oxido(dioxo)vanadium
CAS:7803-55-6 |
|
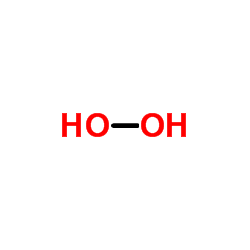 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
titanium dioxide
CAS:13463-67-7 |
|
 |
Titanium oxide
CAS:1317-80-2 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Titanium(IV) oxide, anatase
CAS:1317-70-0 |