| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
Chloroform
CAS:67-66-3 |
|
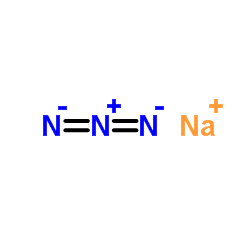 |
Sodium azide
CAS:26628-22-8 |
|
 |
Methanol
CAS:67-56-1 |
|
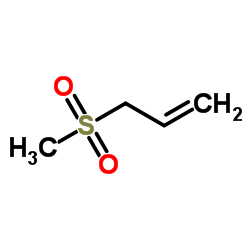 |
Allyl methyl sulfone
CAS:16215-14-8 |
|
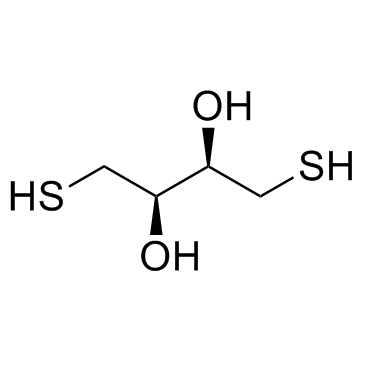 |
DL-Dithiothreitol
CAS:3483-12-3 |