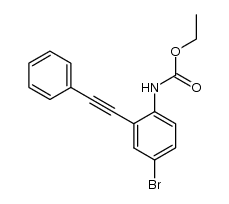
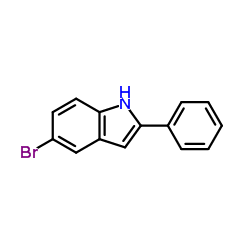
|
~99% |
|
~94% |
|
~49% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~93% |
|
~98% |
|
~% |


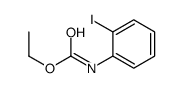
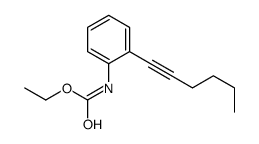
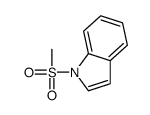
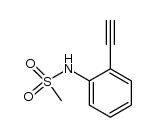
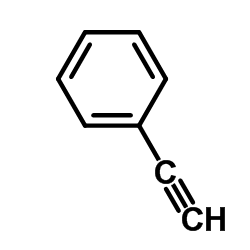

![N-[2-(2-phenylethynyl)phenyl]acetamide Structure](https://image.chemsrc.com/caspic/345/26385-33-1.png)
![N-[2-(2-Phenylethynyl)phenyl]methanamide Structure](https://image.chemsrc.com/caspic/306/13141-39-4.png)