|
~% |
|
~% |
|
~40% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
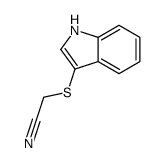
![2-[1-(2-methoxyethyl)indol-3-yl]sulfanylacetonitrile Structure](https://image.chemsrc.com/caspic/117/61021-41-8.png)
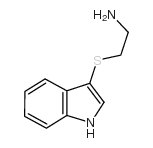
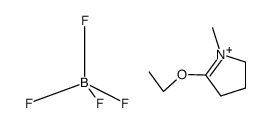
![N-[2-(1H-indol-3-ylsulfanyl)ethyl]-1-methylpyrrolidin-2-imine Structure](https://image.chemsrc.com/caspic/256/61020-73-3.png)

![N-[3-(1H-indol-3-ylsulfanyl)propyl]-1-methylpyrrolidin-2-imine Structure](https://image.chemsrc.com/caspic/180/61020-75-5.png)
![2-[1-(cyclopropylmethyl)indol-3-yl]sulfanylacetonitrile Structure](https://image.chemsrc.com/caspic/079/61021-42-9.png)
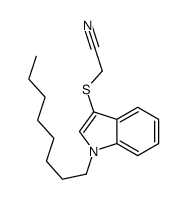
![2-[1-(furan-2-ylmethyl)indol-3-yl]sulfanylacetonitrile Structure](https://image.chemsrc.com/caspic/493/61021-44-1.png)
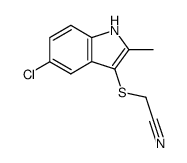
![2-[(5-chloro-2-methyl-1H-indol-3-yl)sulfanyl]ethanamine Structure](https://image.chemsrc.com/caspic/246/83748-17-8.png)
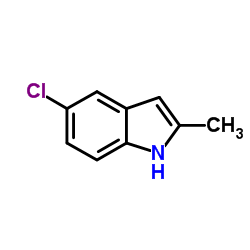
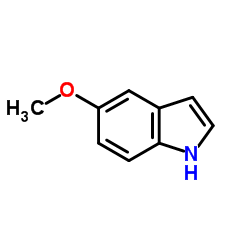
![2-[(5-methoxy-1H-indol-3-yl)sulfanyl]acetonitrile Structure](https://image.chemsrc.com/caspic/324/61021-31-6.png)
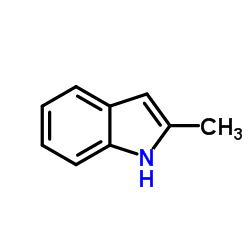
![2-[(2-methyl-1H-indol-3-yl)sulfanyl]acetonitrile Structure](https://image.chemsrc.com/caspic/286/61021-32-7.png)

![2-[(5-chloro-1H-indol-3-yl)sulfanyl]acetonitrile Structure](https://image.chemsrc.com/caspic/248/61021-33-8.png)
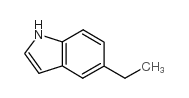
![2-[(5-ethyl-1H-indol-3-yl)sulfanyl]acetonitrile Structure](https://image.chemsrc.com/caspic/210/61021-34-9.png)
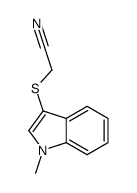
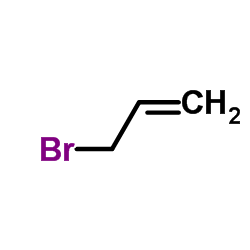
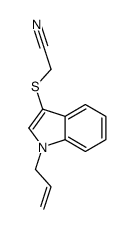
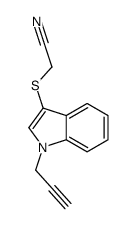
![2-[1-(2-methylprop-2-enyl)indol-3-yl]sulfanylacetonitrile Structure](https://image.chemsrc.com/caspic/379/61021-47-4.png)
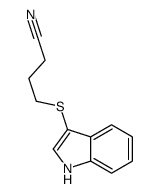

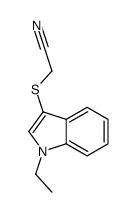
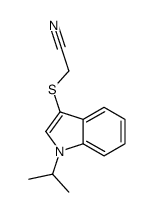
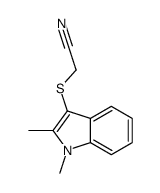
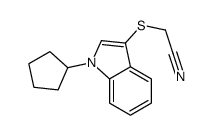
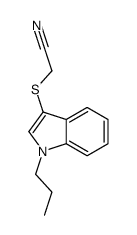
![2-[(2-METHYL-1H-INDOL-3-YL)THIO]ETHANAMINE Structure](https://image.chemsrc.com/caspic/003/61021-66-7.png)