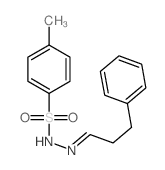
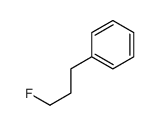
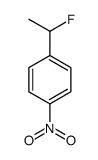
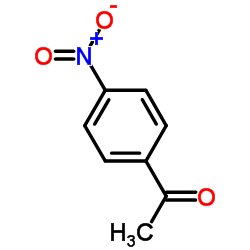
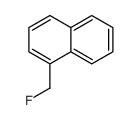
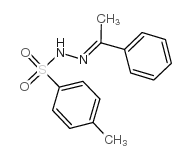
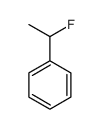
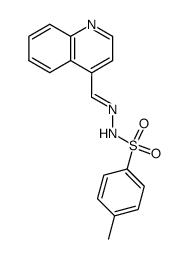
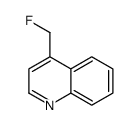
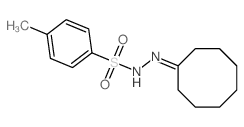
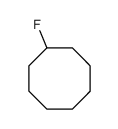
|
~78% |
|
~79% |
|
~62% |
|
~% |
|
~88% |
|
~85% |
|
~75% |
|
~81% |
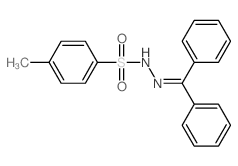
![[fluoro(phenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/298/579-55-5.png)