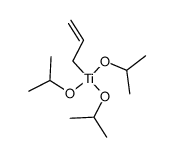
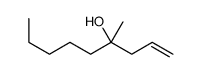
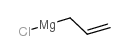
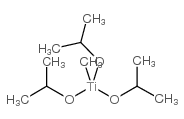
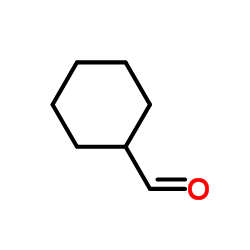
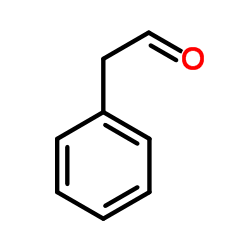
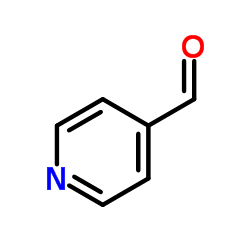
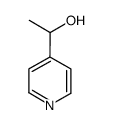
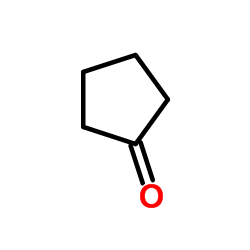
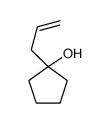
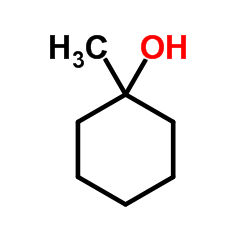
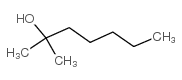
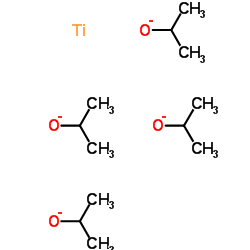
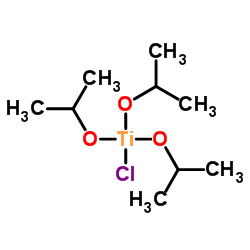
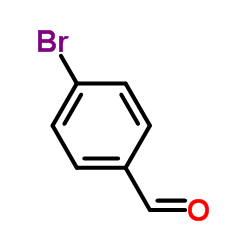
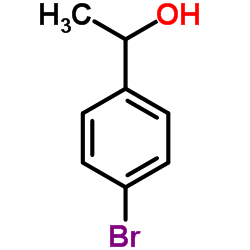
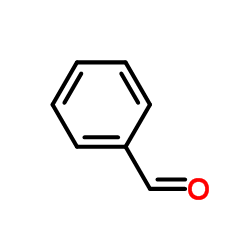
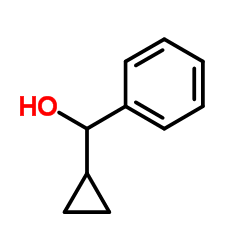
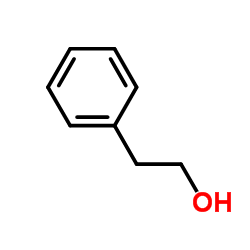
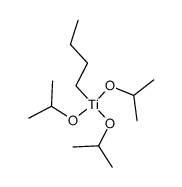
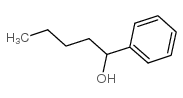
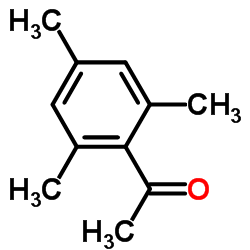
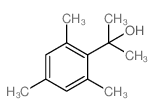
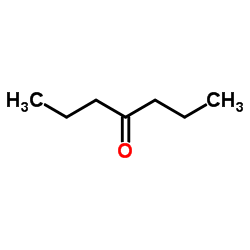
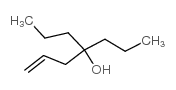
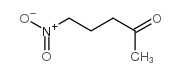
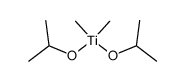
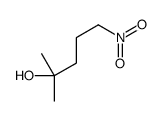
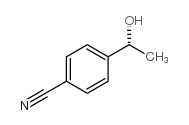
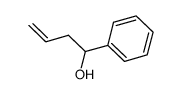
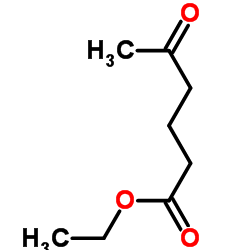
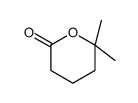
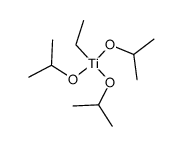
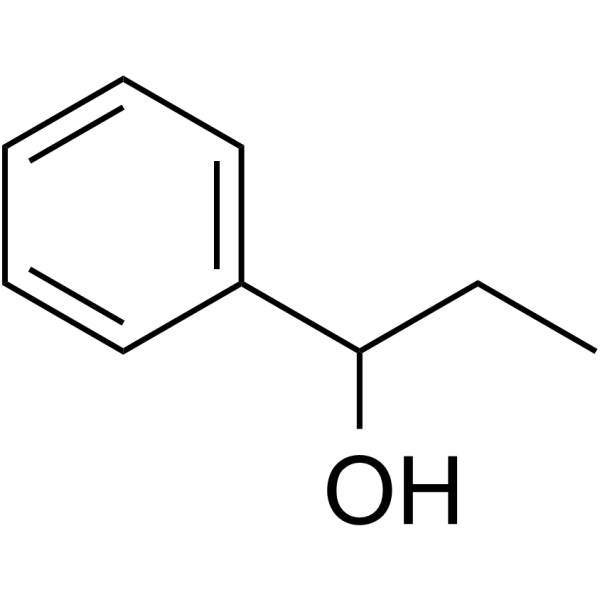
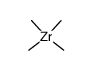|
~78% |
|
~% |
|
~84% |
|
~71% |
|
~75% |
|
~% |
|
~% |
|
~80% |
|
~80% |
|
~97% |
|
~94% |
|
~70% |
|
~91% |
|
~74% |
|
~28% |
|
~81% |
|
~% |
|
~% |
|
~78% |
|
~14% |
|
~79% |
|
~83% |
|
~84% |
|
~55% |
|
~71% |