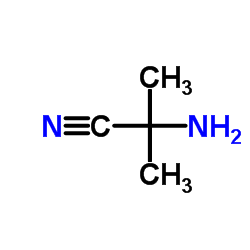
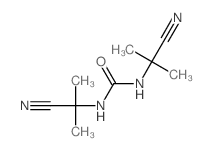
|
~% |
|
~% |
|
~% |
|
~% |
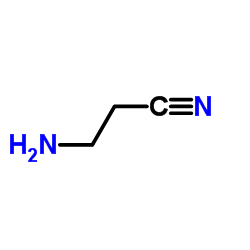


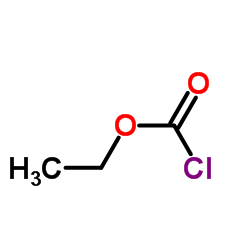

![2-imino-3,3,7,7-tetramethyl-2,3,6,7-tetrahydro-imidazo[1,5-a]imidazol-5-one Structure](https://image.chemsrc.com/caspic/316/108483-18-7.png)