|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~68% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

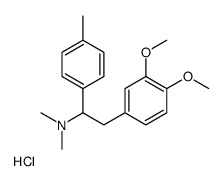
![[2-benzoyloxy-4-[2-(dimethylamino)-2-(4-methylphenyl)ethyl]phenyl] benzoate,hydrochloride Structure](https://image.chemsrc.com/caspic/314/87213-07-8.png)


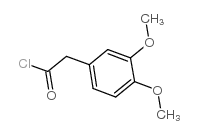
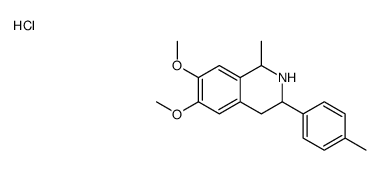
![1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-3-(4-methylphenyl)-1,2,3,4-tetrahydroisoquinoline,hydrochloride Structure](https://image.chemsrc.com/caspic/322/87203-95-0.png)
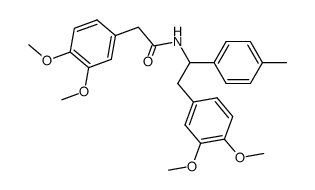
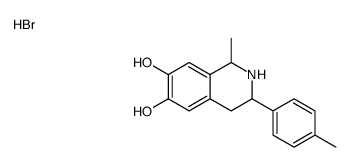
![1-[2-(3,4-dimethoxyphenyl)-1-(4-methylphenyl)ethyl]-4-methylpiperazine,dihydrochloride Structure](https://image.chemsrc.com/caspic/084/87203-79-0.png)
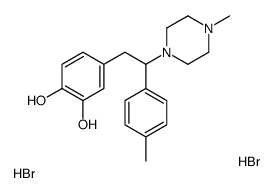
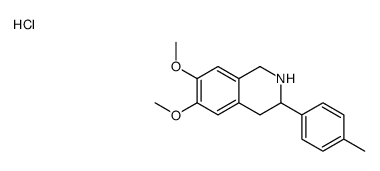
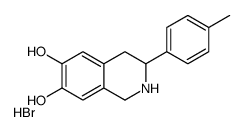
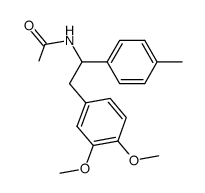
![N-[2-(3,4-dimethoxyphenyl)-1-(4-methylphenyl)ethyl]formamide Structure](https://image.chemsrc.com/caspic/262/65884-32-4.png)

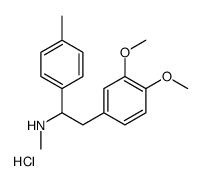
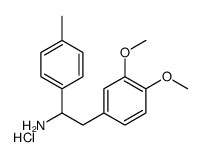
![4-[2-(methylamino)-2-(4-methylphenyl)ethyl]benzene-1,2-diol,hydrobromide Structure](https://image.chemsrc.com/caspic/284/87203-74-5.png)
![1,2-dimethoxy-4-[2-(4-methylphenyl)ethenyl]benzene Structure](https://image.chemsrc.com/caspic/350/24815-56-3.png)
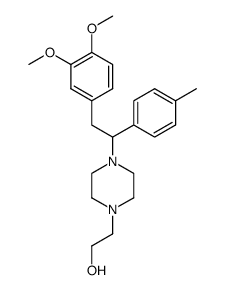
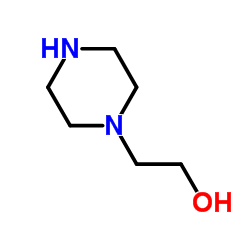
![4-[2-[4-(2-hydroxyethyl)piperazin-1-yl]-2-(4-methylphenyl)ethyl]benzene-1,2-diol,dihydrobromide Structure](https://image.chemsrc.com/caspic/039/87203-85-8.png)