|
~% |
|
~% |
|
~80% |
|
~47% |
|
~% |
|
~% |
|
~% |
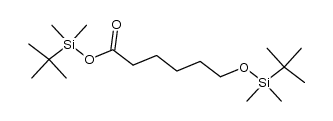
![6-[tert-butyl(dimethyl)silyl]oxyhexanoic acid Structure](https://image.chemsrc.com/caspic/079/77744-44-6.png)
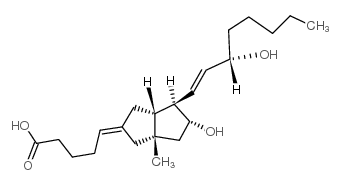
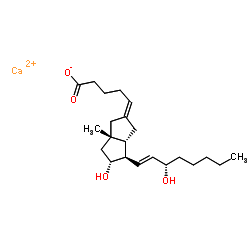
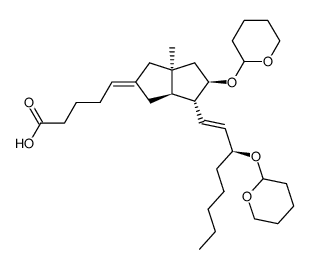

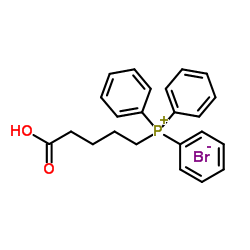
![(3'S)-7α-[(Tetrahydropyran-2-yl)oxy]-6β-[3'-[(tetrahydropyran-2-yl)oxy]-trans-1'-octenyl]-bicyclo[3.3.0]oct-1-en-3-one Structure](https://image.chemsrc.com/caspic/059/76794-02-0.png)
![(3'S)-1β-Methyl-7α-[(tetrahydropyran-2-yl)oxy]-6β-[3'-[(tetrahydropyran-2-yl)oxy]-trans-1'-octenyl]bicyclo[3.3.0]octan-3-one Structure](https://image.chemsrc.com/caspic/487/81845-43-4.png)