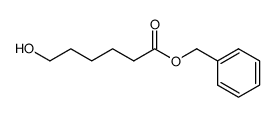
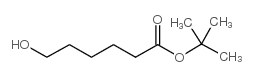
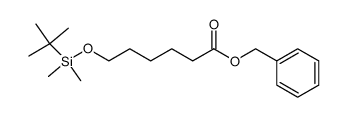
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
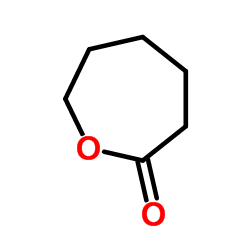
![6-[tert-butyl(dimethyl)silyl]oxyhexanoic acid Structure](https://image.chemsrc.com/caspic/079/77744-44-6.png)