|
~66% |
|
~% |
|
~67% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~31% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~69% |
|
~% |
|
~77% |
|
~65% |
|
~% |
|
~% |
|
~53% |
|
~57% |
|
~46% |
|
~88% |
|
~% |
|
~49% |
|
~% |
|
~% |
|
~26% |
|
~51% |
|
~% |
|
~54% |
|
~38% |
|
~% |
|
~86% |
|
~% |
|
~8% |

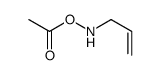
![[(2-cyclopent-2-en-1-ylacetyl)-prop-2-enylamino] acetate Structure](https://image.chemsrc.com/caspic/210/77413-82-2.png)
![[acetyl(prop-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/151/87842-65-7.png)
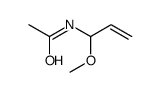
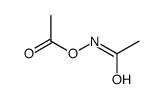
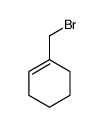
![[acetyl(cyclohexen-1-ylmethyl)amino] acetate Structure](https://image.chemsrc.com/caspic/075/87842-89-5.png)
![[pent-4-enoyl(prop-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/151/77413-79-7.png)
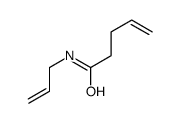
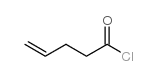
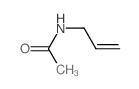
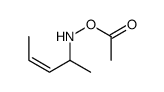
![[pent-4-enoyl(pent-3-en-2-yl)amino] acetate Structure](https://image.chemsrc.com/caspic/058/87842-72-6.png)
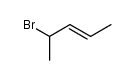
![[acetyl(pent-3-en-2-yl)amino] acetate Structure](https://image.chemsrc.com/caspic/151/87842-87-3.png)
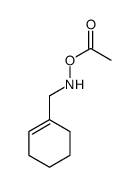
![[cyclohexen-1-ylmethyl(pent-4-enoyl)amino] acetate Structure](https://image.chemsrc.com/caspic/358/87842-77-1.png)
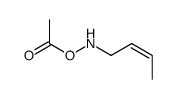
![[but-2-enyl(pent-4-enoyl)amino] acetate Structure](https://image.chemsrc.com/caspic/265/87842-84-0.png)
![[acetyl(but-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/227/87842-85-1.png)
![[hex-5-enoyl(prop-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/286/77413-80-0.png)
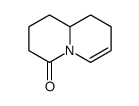

![methyl [pent-4-enoyl(prop-2-enyl)amino] carbonate Structure](https://image.chemsrc.com/caspic/489/87842-69-1.png)



![[prop-2-enoxycarbonyl(prop-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/227/87842-63-5.png)
![[pent-4-ynoyl(prop-2-enyl)amino] acetate Structure](https://image.chemsrc.com/caspic/189/87842-64-6.png)