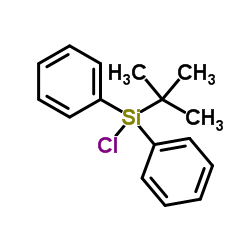
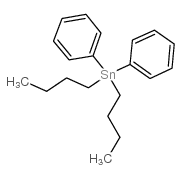
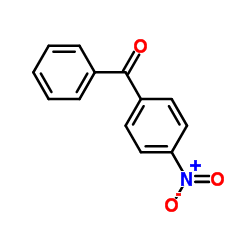
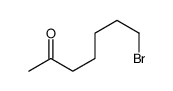
|
~70% |
|
~84% |
|
~90% |
|
~82% |
|
~64% |
|
~64% |
|
~87% |
|
~97% |
|
~87% |
|
~86% |
|
~60% |
|
~% |
|
~% |
|
~79% |
|
~98% |
|
~46% |
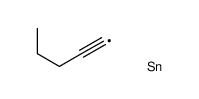

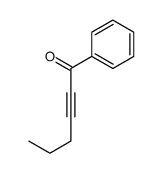
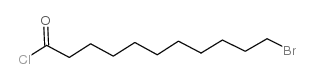
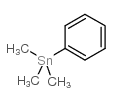

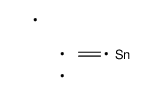
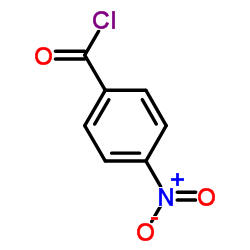
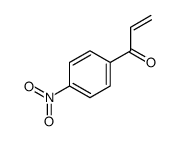

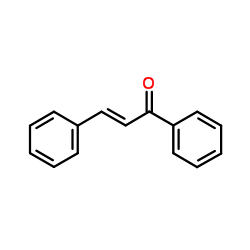
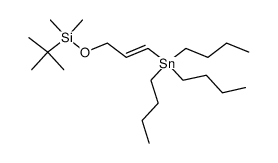
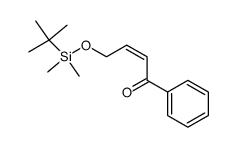
![4-[tert-butyl(dimethyl)silyl]oxy-1-phenylbut-2-en-1-one Structure](https://image.chemsrc.com/caspic/036/87305-71-3.png)
![TRIBUTYL[3-(TRIFLUOROMETHYL)PHENYL]STANNANE Structure](https://image.chemsrc.com/caspic/190/53566-38-4.png)

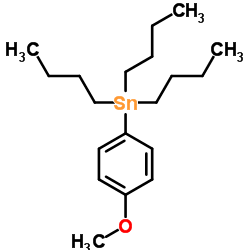
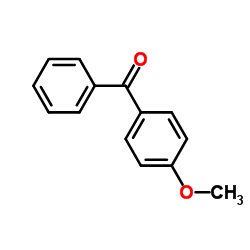

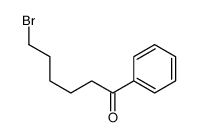
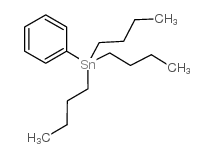

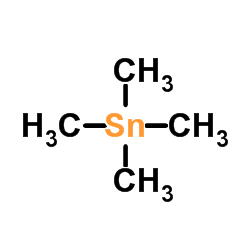

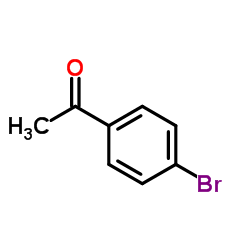

![4-[tert-butyl(diphenyl)silyl]oxypentanoyl chloride Structure](https://image.chemsrc.com/caspic/477/87305-68-8.png)