|
~62% |
|
~% |
|
~65% |
|
~% |
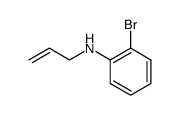

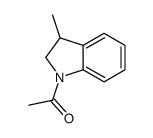

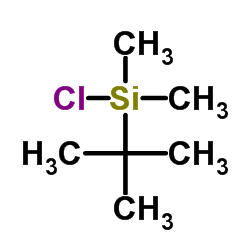
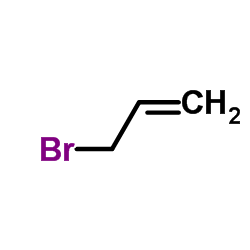
![trimethyl-[(1-prop-2-enyl-2,3-dihydroindol-3-yl)methyl]silane Structure](https://image.chemsrc.com/caspic/279/176376-90-2.png)