|
~% |
|
~% |
|
~% |
|
~% |
|
~0%
Detail
|
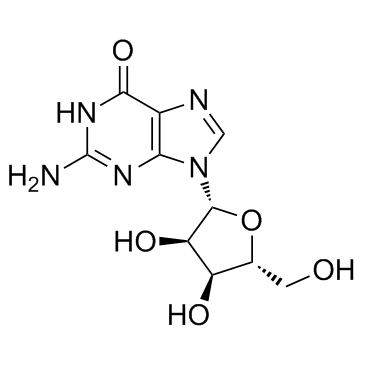
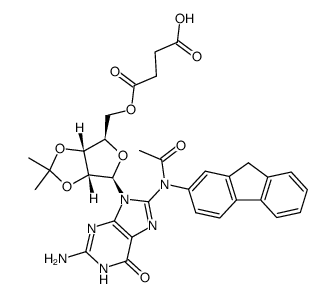
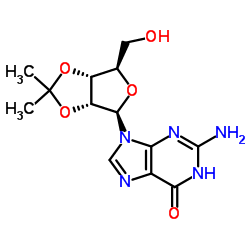
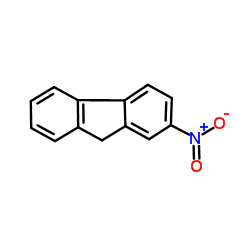

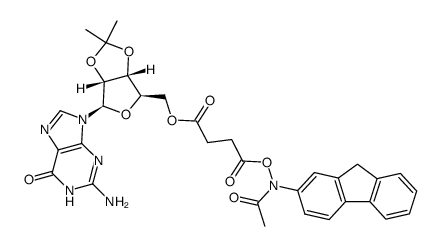

![2-acetamido-9H-fluoren-1-yl (((3aR,4R,6R,6aR)-6-(2-amino-6-oxo-1H-purin-9(6H)-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl) succinate Structure](https://image.chemsrc.com/caspic/024/137390-97-7.png)