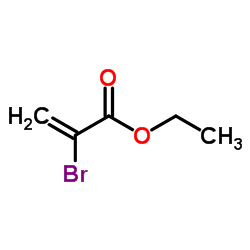
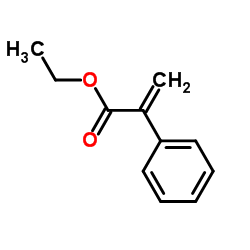
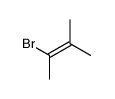
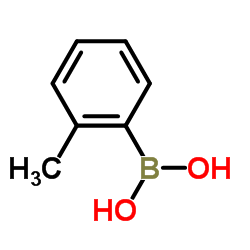
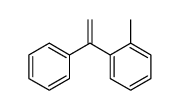
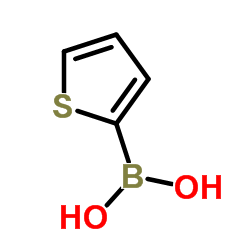
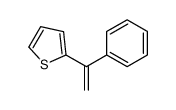
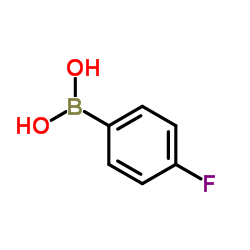
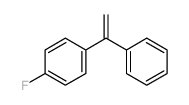
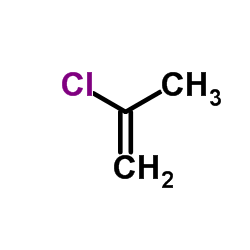
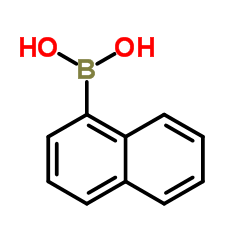
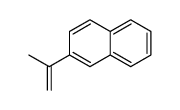
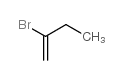
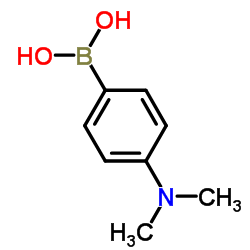
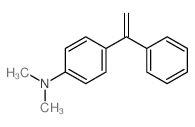
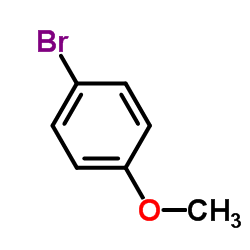
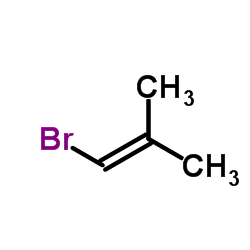
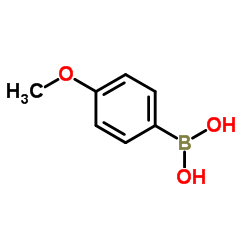
|
~67% |
|
~% |
|
~% |
|
~97% |
|
~77% |
|
~83% |
|
~85% |
|
~77% |
|
~85% |
|
~55% |
|
~84% |
|
~78% |
|
~93% |
|
~89% |
|
~60% |
|
~% |
|
~% |
|
~86% |

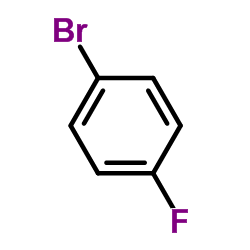
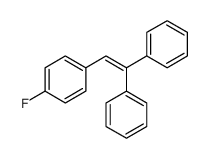
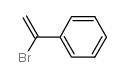
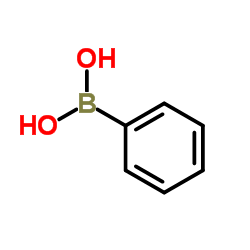
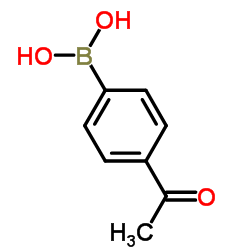
![1-[4-(1-phenylethenyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/132/87324-13-8.png)