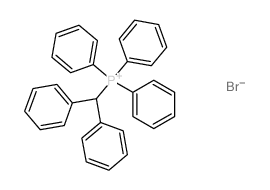
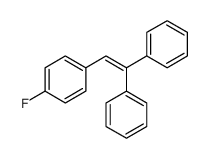
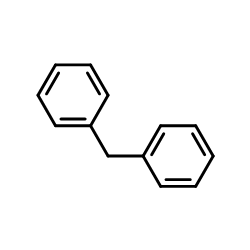
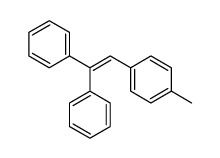
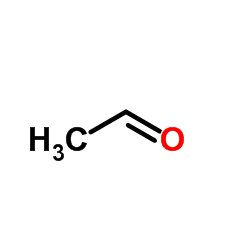
|
~63% |
|
~56% |
|
~94% |
|
~73% |