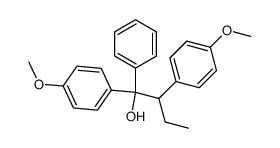
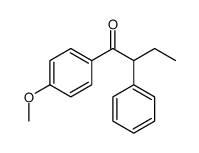
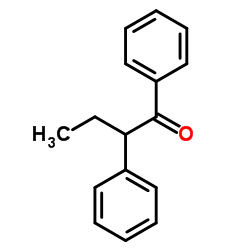
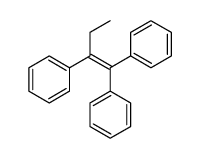
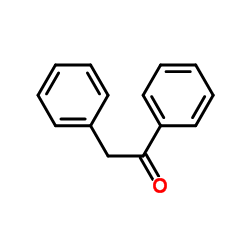
|
~38% |
|
~% |
|
~% |
|
~36% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
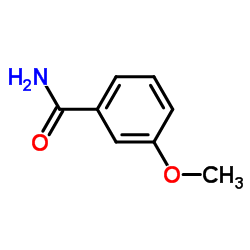
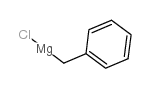
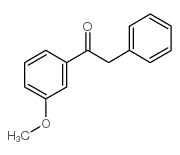
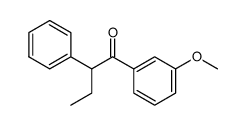
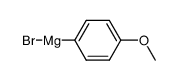
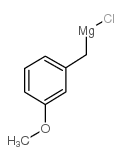

![1-methoxy-4-[1-(4-methoxyphenyl)-2-phenylbut-1-enyl]benzene Structure](https://image.chemsrc.com/caspic/424/82333-56-0.png)
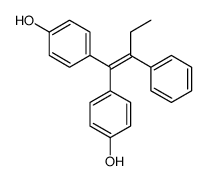
![4-[1-(4-hydroxyphenyl)-1-phenylbut-1-en-2-yl]phenol Structure](https://image.chemsrc.com/caspic/426/4120-45-0.png)