3-Methoxybenzamide
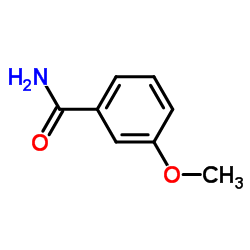
3-Methoxybenzamide structure
|
Common Name | 3-Methoxybenzamide | ||
|---|---|---|---|---|
| CAS Number | 5813-86-5 | Molecular Weight | 151.163 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 280.0±23.0 °C at 760 mmHg | |
| Molecular Formula | C8H9NO2 | Melting Point | 132.5-135.5 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 146.8±18.9 °C | |
Use of 3-Methoxybenzamide3-Methoxybenzamide (3-MBA), an inhibitor of ADP-ribosyltransferase (ADPRTs) and PARP, inhibits cell division in Bacillus subtilis, leading to filamentation and eventually lysis of cells[1]. 3-Methoxybenzamide (3-MBA) enhances in vitro plant growth, microtuberization, and transformation efficiency of blue potato (Solanum tuberosum L. subsp. andigenum)[2]. |
| Name | 3-methoxybenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Methoxybenzamide (3-MBA), an inhibitor of ADP-ribosyltransferase (ADPRTs) and PARP, inhibits cell division in Bacillus subtilis, leading to filamentation and eventually lysis of cells[1]. 3-Methoxybenzamide (3-MBA) enhances in vitro plant growth, microtuberization, and transformation efficiency of blue potato (Solanum tuberosum L. subsp. andigenum)[2]. |
|---|---|
| Related Catalog | |
| In Vitro | 3-Methoxybenzamide (0-30 mM) affects septation via the FtsZ system during both vegetative growth and sporulation[1]. 3-Methoxybenzamide (3 MB; 0.2 to 0.6 mM) significantly increaseS the growth and microtuber formation of in vitro propagated plants[2]. Cell Viability Assay[1] Cell Line: ftsZ mutant strains. Concentration: 0-30 mM. Incubation Time: 30℃ overnight. Result: Changes directly or indirectly the ability of ftsZ to bind or hydrolyze the guanine nucleotide, ultimately leading to the inhibition of Zring formation. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 280.0±23.0 °C at 760 mmHg |
| Melting Point | 132.5-135.5 °C(lit.) |
| Molecular Formula | C8H9NO2 |
| Molecular Weight | 151.163 |
| Flash Point | 146.8±18.9 °C |
| Exact Mass | 151.063324 |
| PSA | 52.32000 |
| LogP | 0.85 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.546 |
| InChIKey | VKPLPDIMEREJJF-UHFFFAOYSA-N |
| SMILES | COc1cccc(C(N)=O)c1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CV5463333 |
| HS Code | 2924299090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Characterization of the superinduction of the c-myc proto-oncogene in fibroblasts by benzamide derivatives.
Mol. Cell Biochem. 124(2) , 175-81, (1993) In mouse fibroblasts stimulated from quiescence into proliferation by serum the induction of expression of the c-myc proto-oncogene is strongly stimulated by 3-methoxybenzamide. Similar superinduction... |
|
|
Immunosuppressive activities of 6(5H)-phenanthridinone, a new poly(ADP-ribose)polymerase inhibitor.
Int. J. Immunopharmacol. 17(4) , 265-71, (1995) 6(5H)-phenanthridinone, a recently identified poly(ADP-ribose)polymerase (PARP) inhibitor, is able, at micromolar concentrations, to inhibit concanavalin A-induced lymphocyte proliferation and to pote... |
|
|
Antibacterial alkoxybenzamide inhibitors of the essential bacterial cell division protein FtsZ.
Bioorg. Med. Chem. Lett. 19 , 524-7, (2009) 3-Methoxybenzamide is a weak inhibitor of the essential bacterial cell division protein FtsZ. Exploration of the structure-activity relationships of 3-methoxybenzamide analogues led to the identificat... |
| 3-Methoxybenzamide |
| MFCD00007986 |
| 3-Methoxy-benzoesaeure-amid |
| 3-Methoxy-benzamid |
| 3-methoxy-benzoic acid amide |
| m-anisamide |
| Benzamide, 3-methoxy- |
| Benzamide,3-methoxy |
| 3-Methoxy-benzamide |
| EINECS 227-379-7 |
| 3-Methoxyphenylformamide |
| m-Methoxybenzamide |
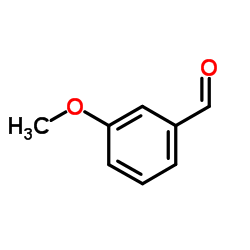 CAS#:591-31-1
CAS#:591-31-1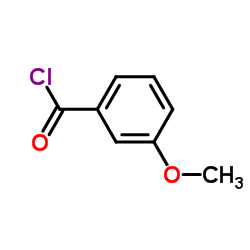 CAS#:1711-05-3
CAS#:1711-05-3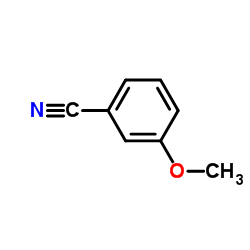 CAS#:1527-89-5
CAS#:1527-89-5 CAS#:5071-96-5
CAS#:5071-96-5 CAS#:38489-80-4
CAS#:38489-80-4 CAS#:6971-51-3
CAS#:6971-51-3 CAS#:586-37-8
CAS#:586-37-8 CAS#:586-38-9
CAS#:586-38-9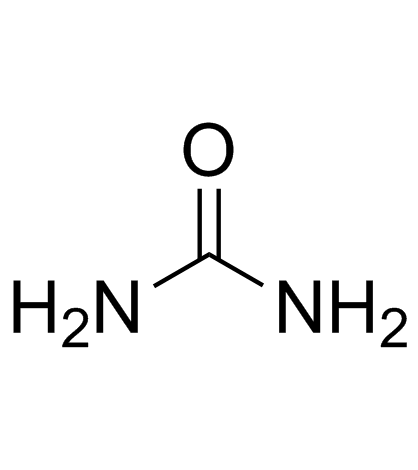 CAS#:57-13-6
CAS#:57-13-6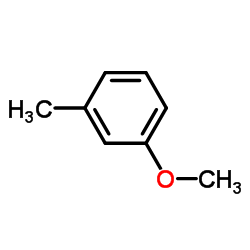 CAS#:100-84-5
CAS#:100-84-5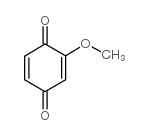 CAS#:2880-58-2
CAS#:2880-58-2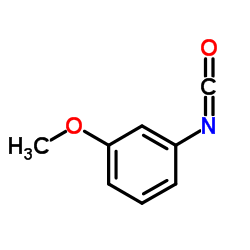 CAS#:18908-07-1
CAS#:18908-07-1 CAS#:618-49-5
CAS#:618-49-5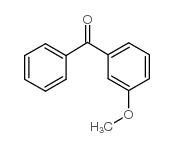 CAS#:6136-67-0
CAS#:6136-67-0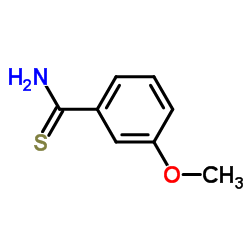 CAS#:64559-06-4
CAS#:64559-06-4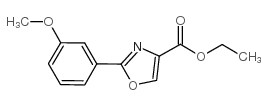 CAS#:132089-44-2
CAS#:132089-44-2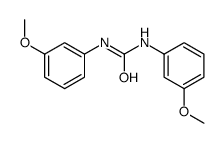 CAS#:16460-28-9
CAS#:16460-28-9
